Electrodialysis (ED) is a very versatile technology for the separation of difficult mixtures. Guo Chu technology (Xiamen) Co., Ltd. offers expertise in electrodialysis R&D as well as in the engineering and construction of ED systems for the laboratory, piloting, and production.
What is Electrodialysis (ED)?
Electrodialysis is an electromembrane process in which ions are transported through ion permeable membranes from one solution to another under the influence of a potential gradient. The electrical charges on the ions allow them to be driven through the membranes fabricated from ion exchange polymers. Applying a voltage between two end electrodes can generate the required potential field. Since the membranes used in electrodialysis have the ability to selectively transport ions having positive or negative charge and reject ions of the opposite charge. Therefore, electrodialysis can achieve effective concentration, removal or separation of electrolytes.
The ion exchange membranes used in electrodialysis are essentially sheets of ion-exchange resins. They usually also contain other polymers to improve mechanical strength and flexibility. The resin component of a cation-exchange membrane would have negatively charged groups (e.g., -SO3–) chemically attached to the polymer chains (e.g., styrene/divinylbenzene copolymers). Ions with a charge opposite to the fixed charge (counter ions) are freely exchanged at these sites. The concentration of counter ions (e.g., Na+) is relatively high, therefore, counter ions carry most of the electric current through the membrane. The fixed charges attached to the polymer chains repel ions of the same charge (co-ions), in this case the anions. Since their concentration in the membrane is relatively low, anions carry only a small fraction of the electric current through a cation permeable membrane. Attachment of positive fixed charges (e.g., -NR3+ or C5H5N+R where commonly R = CH3) to the polymer chains forms anion exchange membranes, which are selective to transport of negative ions, because the fixed -NR3+ groups repel positive ions. This exclusion, as a result of electrostatic repulsion, is called Donnan exclusion.
Ion-exchange polymers such as poly(styrene sulfonic acid) are water soluble, so crosslinking is needed to prevent dissolution of ion permeable membranes. Divinylbenzene is used to cross link polystyrene chains. The degree of cross-linking and the fixed-charge density affect the membrane’s properties in opposite ways. Higher crosslinking improves selectivity and membrane stability by reducing swelling, but it increases electrical resistance. High charge density reduces resistance and increases selectivity, but it promotes swelling and thus necessitates higher crosslinking. A compromise between selectivity, electrical resistance, and dimensional stability is achieved by proper adjustment of crosslinking and fixed-charge densities.
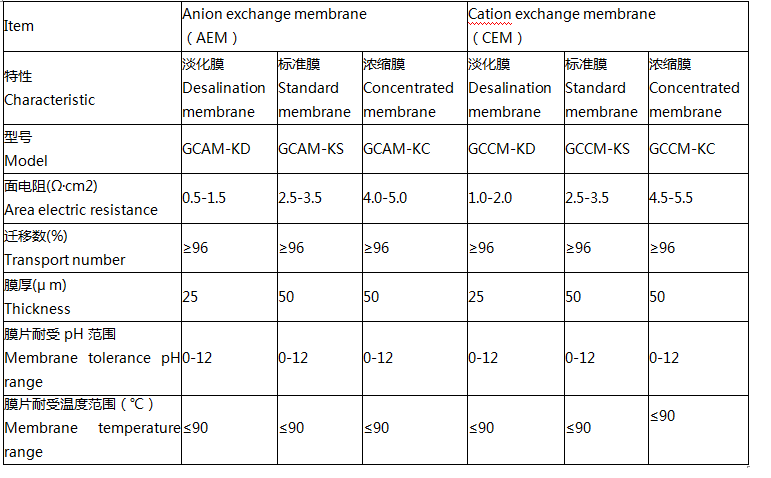
Product parameters
The following are our electrodialysis modules from small to industrial scale.

We can also provide complete sets of experimental and industrial devices.




 Ion Exchange Membranes Applications
Reduce Electrolyte Content:
Ion Exchange Membranes Applications
Reduce Electrolyte Content:
Potable from brackish water
Food products – whey, milk, soy sauce, fruit juice
Nitrate from drinking water
Cooling tower water
Boiler feed water
Rinse water for electronics processing
Electroless plating baths
Recovery of blood plasma proteins
Pickle brines to recover flavor
Sugar and molasses
Amino acids
Potassium tartrate from wine
Chloride purge in Kraft paper process
Photographic developer regeneration
Fiber reactive dyes
Recover Electrolytes:
Pure NaCl from seawater
Ag(I) salts from photographic waste
Ni(II) from electroplating rinse water
Zn(II) from galvanizing rinse water
Salts of organic acids from fermentation broth
Amino acids from protein hydrolysates
Acids from metal pickling baths and rinse
HCl from cellulose hydrolysate
Miscellaneous:
Salt splitting
Metathesis
Concentrate reverse osmosis brines
Ion substitution
As you can see, the ion exchange membranes have a wide range of applications in many fields. Our experience with ion exchange membranes can help you determine whether it is the appropriate technology for your needs.
Contact:
Tel:+86 592 6514970
Email:market@guochukeji.com
Skype:18060902001
WhatsApp:18060902001
WeChat:18060902001
TM: 18060902001
QQ:1641011431